|
Dynamic response of decrease in Salmonella spp. in the cecum of chickens: Impact of using mananooligosaccharides and β-glucans (1,3) (1,6)
John Jairo Salazar1 and Luis Miguel Mejía2
1 John Jairo Salazar MVZ, MSc. Immunopathology Laboratory, Colombia. 2 Biostatistics Laboratory, Universidad del Quindío, Colombia.
This email address is being protected from spambots. You need JavaScript enabled to view it.
Recibido: 22 agosto de 2019, Aprobado: 27 noviembre de 2019, actualizado: 19 diciembre de 2019
DOI: 10.17151/vetzo.2020.14.1.8
ABSTRACT. Introduction: Salmonella spp. is classified as a Gram-negative bacterium and is characterized as a facultative anaerobe. The worldwide prevalence of Salmonella enteritidis is approximately 100 million cases. Aim: To study the effect of mananooligosaccharides and β-glucans (1,3) (1,6) for stimulating cellular immune response at the gastrointestinal level in the cecum of chickens to reduce Salmonella spp. colonization. Methods: A systematic review and meta-analysis were performed, including studies performed between 1980 and 2020. A total of 16 research papers with 28 treatments were chosen, and the statistical software Stat Graphics Stratus was used. Commands comparing the test with multiple ranges were used; groups treated with β-glucans (1,3) (1,6) in the cecum were used for determining the prevalence of Salmonella enteritidis, and groups not treated with β-glucans were used as control. The significance (P > 0.05) was validated by Tukey’s test and the corresponding linear regression equation was obtained. Results: There were differences between the prevalence of cecum-contaminated chickens in the β-glucan (1,3) (1,6) treated group and the control group. A mean of 29.60 ± 4.84 SD was observed in the treated group vs. 62.52 ± 47.6 SD in the control group (P = 0.0000), based on which it can be concluded that there is a highly significant difference in favor of the group treated with β-glucans (1,3) (1,6). A statistical contrast test was performed over the differences, which yielded a result in favor of using β-glucans (1,3) (1,6) because they decrease Salmonella spp. contamination of the cecum by 32.93 ± 13.3%. Conclusions: β-Glucans (1,3) (1,6) can be used to reduce Salmonella spp. colonization in the cecum of chickens, which would consequently decrease the systemic transfer of this bacterium to other organs, especially to eggs that are for human consumption.
Key Words: β-glucans, mananooligosaccharides, Salmonella spp.
Respuesta dinámica de la disminución de Salmonella spp en el ciego de las aves, impacto del uso de mananooligosacaridos y Β-glucanos (1,3)(1,6)
RESUMEN. Introducción: La Salmonella spp está clasificada como una bacteria Gram negativa, se caracteriza por ser anaeróbia facultativa, la prevalencia mundial de la Salmonella enteritidis se ha calculado en aproximadamente cien millones de casos, Objetivos: investigar el efecto que poseen los mananooligosacaridos y los β-glucanos (1,3)(1,6) al estimular la respuesta inmune celular a nivel gastrointestinal en el ciego de las aves, disminuyendo la contaminación por Salmonella spp, Métodos: Se realizó una revisión sistemática y meta-análisis, donde se incluyeron investigaciones realizadas entre los años 1980 y 2020, Se escogieron dieciséis trabajos de investigación con veintiocho tratamientos, se utilizó el programa estadístico Stat Graphics Stratus, Se usaron los comandos que comparan la prueba con múltiples rangos, para prevalencias de Salmonella, grupos intervenidos con β-glucanos (1,3)(1,6) y grupos control sin β-glucanos en el ciego, La prueba de significancia (P<0,05) validándolo con la prueba Tukey y se construyó la ecuación de regresión lineal correspondiente, Resultados: hubo diferencias entre la prevalencia de aves contaminadas en el ciego del grupo tratado con β-glucanos (1,3)(1,6) con las del grupo control no tratado; arrojo una media de 29,60 +/- 4,84 DS del grupo intervenido Vs 62,52 +/- 47,6 DS para el grupo control, con un valor de P=0,0000, donde se concluye que se presenta una diferencia altamente significativa a favor del grupo donde se utilizaron los β-glucanos (1,3)(1,6), Se realizó la prueba estadística contraste de las diferencias, la cual mostro resultado a favor de usar β-glucanos (1,3)(1,6) ya que disminuye en un 32,93% +/- la contaminación por Salmonella spp en el ciego de las aves con un límite de variación del 13,3%, Conclusiones: se pueden usar β-glucanos (1,3)(1,6), para disminuir la colonización por Salmonella spp en el ciego de aves, que consecuentemente disminuirá el traspaso vía sistémica a otros órganos y especialmente al huevo para consumo humano.
Palabras Claves: β-glucanos, Mananoligosacaridos, Salmonella spp
Introduction
What has been proved need not be discussed with God Pierre-Simón Laplace (1742-1827)
Salmonella spp. is classified as a Gram-negative bacterium and is characterized as a facultative anaerobe. The worldwide prevalence of Salmonella enteritidis (SE) has been calculated to be approximately 100 million cases (Majowicz et al., 2010). In 2000, its annual incidence was estimated to be 5 cases per 100,000 individuals, of which 1.7% cases are of children infected by Salmonella spp. and experiencing acute diarrhea as a symptom. Currently, Salmonella spp. is the second leading cause (following Campylobacter spp.) of foodborne infections in Europe. Within this distribution, evidence has suggested that poultry products (eggs, egg subproducts, and bakery products) were the main cause of these zoonotic diseases, accounting for 45.6% of Salmonella spp. infections and 47.2% of SE infections. Additionally, approximately 50% of these cases were caused by an infected egg, which confirms the aforementioned (Khan-Mohammed et al., 2005). At the beginning of 2020, the United States Center for Disease Control and Prevention reported 1.3 million infected individuals, 26,500 hospitalizations, and 420 deaths.
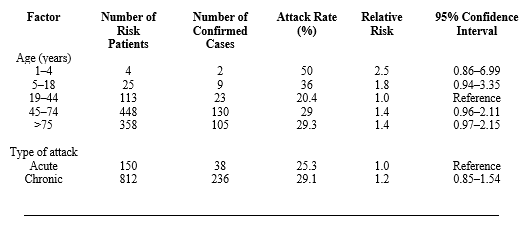
Epidemiology of Salmonella spp. in eggs Epidemiological research identifies consumption of SE-infected eggs from commercial egg farms as the cause of outbreaks (Telzak et al., 1990; Mishu et al., 1991). Telzak et al. (1990) used epidemiology as a prospective tool by taking relative risk as a measure and reported that for each adult consuming SE-infected eggs, the possibility of infecting children aged <4 years is 2.5 fold higher (Table 1).
Table 1. Relative risk of Salmonella spp. infection in hospitals according to age. Source: Telzak et al., 1990
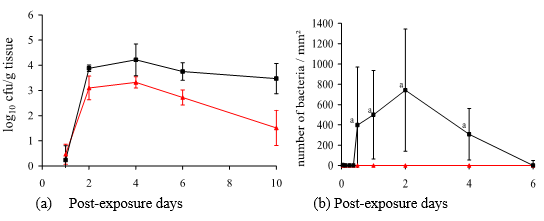
SE in eggs It has been observed that egg yolk and albumin test positive for SE in chickens that are infected naturally and experimentally (Gast, R.K. and Beard, C.W., 1990). These authors showed that following exposure of healthy chickens to the ones infected orally, the eggs of healthy chickens were infected with SE within 7–11 days after inoculation. This suggests bacterial translocation from the cecum to the ovaries (yolk and albumin). Research conducted to support such translocation shows that SE-infected chickens contaminated 15% eggs 2 weeks after inoculation (Timoney, J. F., 1989), based on which the following question arises: what is the bacterial load (number of bacteria) required for SE translocation to the eggs that will eventually be consumed by humans? Humprey (1990) proved that a 1000 cfu/mL dose in chickens exposed orally is the minimum amount necessary for infecting an egg. However, it was also evidenced that on the second day following SE infection, the number of bacteria was 4 log 10 (10,000 cfu/g) in the liver and 800 per mm2 (Figure 1) in the cecum (Immerseel, V. F., 2002).
Figure 1. (a) Salmonella enteritidis colonization in spleen () and liver (∆) of chickens; (b) Number of Salmonella enteritidis in the cecum () of chickens (Immerseel, V.F., 2002).
Salmonella spp. pathogenesis Chickens usually get infected orally when consuming material contaminated with bacteria; vertical transmission is also considered to be a source of infection (Poppe, C., 2000). SE is known to persist in organs, including the spleen, liver, gallbladder, and cecum. Pathogenesis has been divided into the intestinal phase and the systemic infection phase, both regulated by the pathogenicity of Salmonella pathogenicity island gene (SPI-1 and SPI-2) serotypes (Desmidt et al., 1997).
Pathogenesis of the intestinal phase:
A. SE interacts with enterocytes and introduces its SOP proteins into the enterocyte’s cytoplasm through the SPI-1 pathway. B. SOP proteins induce enterocyte membrane agglutination and promote Salmonella spp. invasion. C. Intracellular SE reside in the vesicles fused with the membrane; they are likely involved in the translocation via an extracellular-to-intracellular process mediated by SPI-2, which increases SE replication in those vesicles. D. SOP-B proteins influence the effect of inositol phosphate signaling, blocking the closure of chloride channels, which subsequently affects electrolyte transport and fluid secretion. E. SE-infected enterocytes secrete chymosins and prostaglandins, triggering intense inflammation. F. SE-infected epithelial cells release a chemoattractant, induced by bacteria or PEECs, through the enterocyte apical membrane. This molecule stimulates polymorphonuclear transepithelial migration of the enterocytes toward the intestinal lumen. G. Inflammatory infiltration of SE-infected cells occurs. H. SE-infected enterocytes migrate toward the intestinal lumen, resulting in mucosal loss and intestinal villi damage, deteriorating the absorption surface. I. Lymphatic migration of some infected cells occurs, which causes systemic form of the disease and results in colonization of different organs, especially the spleen, liver, and reproductive system (Barrow, P.A., 1999; Wallis, T.S. and Galyov, E.E., 2000).
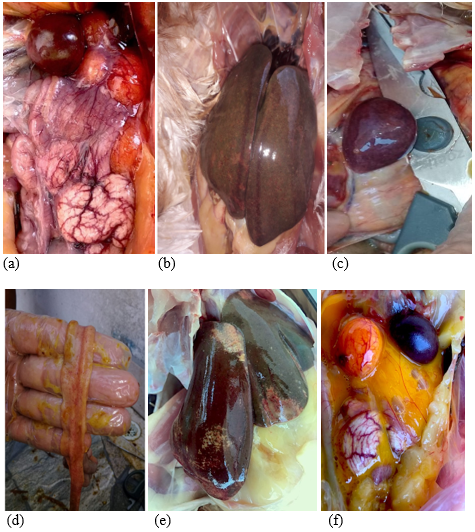
Clinical symptoms include the following: depression, anorexia, drowsiness, low mood, dehydration, dyspnea, frizzled feathers, weakness, adherence of feces in the passageway, and greenish white diarrhea. Macroscopic injuries include the following: splenomegaly, white-mottled spleen, hepatomegaly, and inflammation with necrotic foci. Some chickens may have whitish pericardial and myocardial nodes; additionally, typhlitis with caseous content may be observed in the cecum, along with the loss of intestinal mucosal layer. Ovarian regression; cystic, deformed, and decolorized ovaries; and caseous exudate can be observed in the oviduct (Shivaprasad, H.L., 2000).
Figure 2. (a) Inflammatory peritonitis, S. gallinarum. (b) Hepatomegaly, inflammation with necrotic foci. (c) Mottled splenomegaly. (d) Mucosal detachment. (e) Splenomegaly. (f) Inflammatory egg yolk peritonitis.
Source: John Jairo Salazar (author), Álbum fotográfico Engormix 2019.
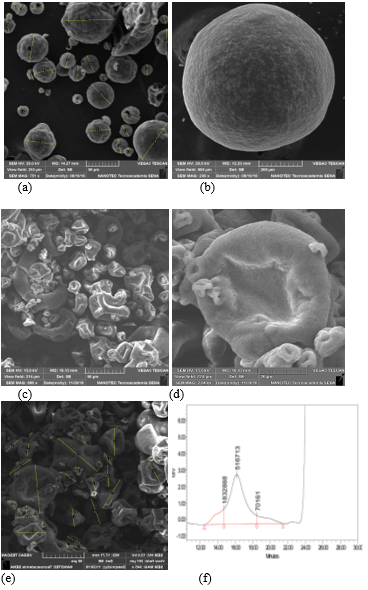
β-glucans (1,3) (1,6) from Saccharomyces cerevisiae β-glucans are glucose polymers forming the cell walls of yeast, bacteria, fungi, and cereal grains, such as oats and barley. β-glucans from yeast and fungal sources are formed by residues of the pyranose group, linked by long 1,3-branches (most) and 1,6-branches (some) (Manners et al., 1973). Yeast β-glucans, obtained as polysaccharides from the S. cerevisiae cell wall, have been widely studied and have reported immunomodulating activity associated with similar recognition receptors, which improves immune response against infectious agents (Saleh et al., 2015; Salazar, J.J., 2019). In vitro and in vivo studies have demonstrated that β-glucans (1,3) (1,6) from the S. cerevisiae cell wall improve innate immunity and humoral immunity in exposed and non-exposed animals (Lowry et al., 2005; Salazar, J.J., 2019).
The bowel has a natural defense mechanism against gastrointestinal pathogens, as it has crypts covered by intestinal villi, mucus, and intestinal epithelium, comprising enterocytes, caliciform cells, Paneth cells, enteroendocrine cells, and stem cells (Rojas et al., 2017). Experiments performed in the countryside have evidenced that chickens exposed to Salmonella thyphimurium had decreased villi length and a negative alteration in the relationship between crypt depth and villi. Chickens exposed to S. thyphimurium, which also received β-glucans (1,3) (1,6), had an increased number of goblet cells in the empty intestine in addition to increased length of intestinal villi (Shao et al., 2013). The reason for use of β-glucans (1,3) (1,6) from S. cerevisiae lies in their capacity to stimulate phagocytosis; macrophages produce an enzyme called iNOS (nitric oxide synthase) as a result of the reaction between nitric acid and superoxide anions, generating toxic components that help macrophages eliminate several types of pathogens, including Salmonella spp. Larry et al. (2005) reported that an iNOS increase is a response to increased β-glucan (1,3) (1,6) administration, which enhances the destructive capacity of macrophages. This was confirmed in 2005 after measurement of the phagocytic index (PI: number of Salmonella spp./number of bacteria phagocytosed). It was demonstrated that chickens fed with β-glucans (1,3) (1,6) for 4 days since birth had PI of 644.1 ± 57 as opposed to 174.54 ± 44 for the control group. This proportion represents a 27% higher capacity of eliminating Salmonella spp. Additionally, β-glucans (1,3) (1,6) are capable of generating local IgA antibodies; the gastrointestinal tract of chickens is an important convergence point between the external environment and the internal body, making gastrointestinal immunity is the first line of defense against several pathogens. In chickens, the gut-associated lymphoid tissue (GAL), comprising cecal tonsils and Peyer’s patches, is an area of lymphocyte aggregation; GAL structures induce an immune response, including IgA production by B cells. IgA, which can be found in the bile and mucosa, neutralizes the virus and bacterial toxins, preventing colonization. IgA levels have been reported to increase in various commercial laying hens exposed to S. thyphimurium; these levels were always higher in chickens receiving β-glucans. Additionally, it is important to state that in this research, S. thyphimurium infiltration levels were reduced in the cecum and liver (Shao et al., 2013; Salazar et al., 2019; Salazar, J.J., 2019).
Most studies have focused on the capacity of β-glucans (1,3) (1,6) to trigger an immunomodulatory effect on cell-mediated immune response, especially by increasing the phagocytic capacity of macrophages and production of IgA antibodies, which reduce Salmonella spp. population in the intestinal lumen. Hence, this research was aimed at determining the capacity of β-glucans to reduce Salmonella spp. population in the cecum, consequently avoiding its translocation into the oviduct by vertical transmission and subsequent contamination of eggs intended for human consumption.
Figure 3. (a) Active dried yeast. (b) Yeast cell wall. (c–e) B-glucans (1,3) (1,6) from Saccharomyces cerevisiae 50 mg. (d) B-glucans (1,3) (1,6). (f) HPLC High performance liquid chromatography, Manno (mannans) GlcN (Glucans). Source: Photographs from the group Levapan-Colombia-NANOTEC SENA 2019.
Materials and Methods
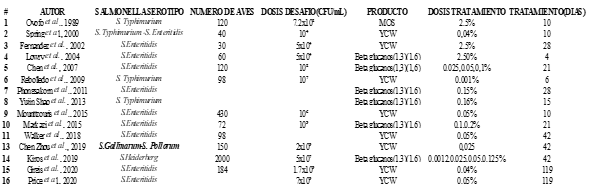
An electronic literature search was performed for the period of 1980–2020, which included all reports written in Spanish and English from NBCI (PMC), US Library of Medicine National Institute, PubMed, Science Direct, and Poultry Science databases. Subsequently, the Quality Consort data extraction model was applied to the selected papers (Farrar, A.M., 2009). Primary search criteria for eligibility were restricted to the following characteristics: author and date of publication, Salmonella serotype, exposure dose (cfu/g or cfu/mL), number of chickens treated with β-glucans, number of chickens in the control group, percentage dose of the product used, and duration of treatment.
Statistical analysis The analysis of variance (ANOVA) was performed on 15 studies, using 28 selected treatments based on the following null hypothesis (H0): the use of β-glucans (1,3) (1,6) reduces bacterial colonization in the cecum of chickens exposed to Salmonella spp.
Statistical model A prevalence variance analysis table was constructed for designing a single factor that is determined randomly. The software used was Stat Graphics Stratus (Excell, 2019). Commands comparing the test with multiple ranges were used for Salmonella prevalence (%) in test groups exposed to β-glucans (1,3) (1,6) and control groups with no β-glucans in the cecum. A significance test was performed (P < 0.05), which was validated using Tukey’s test.
yᵢⅉ= μᵢⅉ + ɛᵢⅉ [25] yᵢⅉ= Response of the ⅉ experimental unit of treatmentᵢ μᵢⅉ= Average response of all experimental units that received treatmentᵢ ɛᵢⅉ= Residual error of the ⅉ unit that received treatmentᵢ
Results
The search performed yielded 101 research papers, classified as follows: 15 scientific reviews, 23 studies with intervention and control groups, 4 books, 15 book chapters, 17 publications in Poultry Science, and 10 publications in Animal Feed Science and Technology. A total of 85 papers were eliminated because they did not have an intervention or a control group or did not estimate standard deviation or error. For statistical validation, 16 research papers were selected, which reported 28 different treatments.
Haga clic sobre la imagen para ampliarla Figure 4. Selected papers.
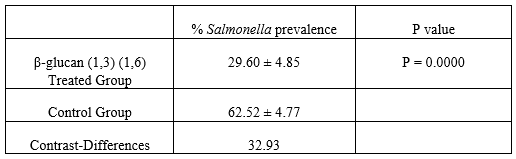
Table 2. Prevalence of Salmonella spp. in groups treated with β-glucans (1,3) (1,6) vs. control group, Tukey’s test. P < 0.05)
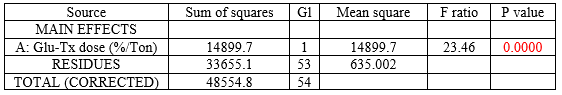
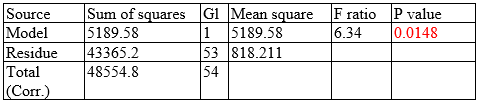
Table 3. Analysis of variance for Salmonella (%)–Type III sum of squares All F ratios are based on mean square of the residual error.
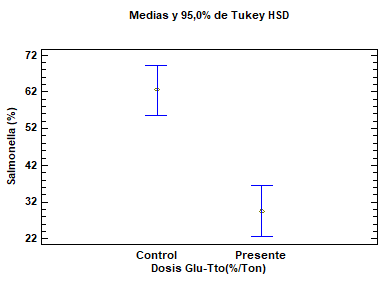
Figure 5. Prevalence of Salmonella spp. in the cecum of chickens treated with β-glucans (1,3) (1,6). Test (β-glucan-treated group). Control (untreated group). Salmonella (%). Salmonella prevalence in the cecum of chickens.
The multiple range test, performed for analyzing differences between the prevalence of chickens with infected cecums in the test group treated with β-glucans (1,3) (1,6) vs. the control group with no treatment, yielded a mean of 29.60 ± 4.84 SD for the treated group vs. 62.52 ± 47.6 SD for the control group (P = 0.0000) (Figure 5) (Tables 2 and 3). Based on these values, a significant difference can be observed in favor of the β-glucan-treated group. A statistical contrast test was performed to confirm the percentage difference between the treated group and the control group, obtaining a result in favor of the use of β-glucans (1,3) (1,6), as colonization of Salmonella spp. in the cecum decreased by 32.93 ± 13.3% (Table 2).
Linear regression (% Prevalence of Salmonella spp.)
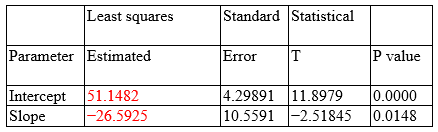
Dependent variable: Salmonella (%) Independent variable: Glu-Tx dose (%Ton) Square root of X: Y = a + b × sqrt(X)
Correlation coefficient = −0.326927 Square R = 10.6881% Square R (degree of freedom adjusted) = 9.00297% Standard error of the est. = 28.6044 Mean absolute error = 23.6461 Durbin–Watson statistical = 1.19372 (P = 0.0006) Residual autocorrelation to lag 1 = 0.397193
The output shows the results of adjusting an X square root model to describe the association between Salmonella (%) and glucan-Tx dose (%Ton). The equation for the adjusted model is as follows:
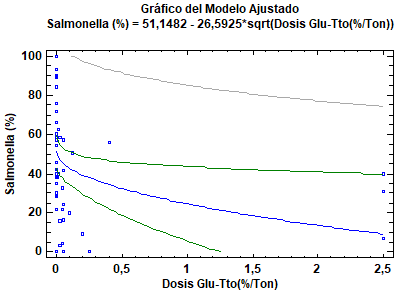
Figure 6. Linear regression (correlation between β-glucan dose and Salmonella spp. prevalence in chicken cecum).
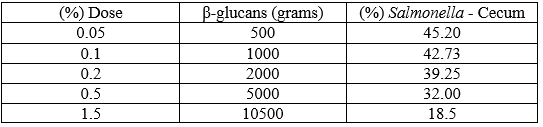
The predicting equation is valid because P = 0.0148 suggests a significant reduction in Salmonella spp. after administration of β-glucans (1,3) (1,6) through food for reducing bacterial colonization in the cecum of chickens (0.0148<0.05). Based on the predicting equation, the prevalence of Salmonella spp. according to each dose for the five scenarios is as follows:
Table 6. Scenarios of Salmonella spp. infection in the cecum based on β-glucan (1,3) (1,6) dose.
Discussion
The term “translocation” is used for defining the passage of pathogenic bacteria from the gastrointestinal tube to the internal organs (Berg, R.D., 1995). Three mechanisms resulting in translocation have been identified: excessive growth of pathogenic bacteria in the cecum (Salmonella spp.), deficient response of the host’s immune system, and intestinal tissue damage that damages the mucosal layer and increases intestinal permeability (Berg, R.D., 1995; Remus et al., 2014; Salazar, J.J., 2019). Remus et al. (2014) demonstrated that bacterial infection of the intestine that deteriorates growth in chickens is distributed as follows: 40% Clostridium spp., 10% Escherichia coli, and 29.9% Salmonella spp. Based on this, we have shown that immunomodulators like β-glucans (1,3) (1,6) can be used because they decrease Salmonella prevalence by 32.93% and activate cellular immune response, which decrease bacterial translocation to the liver and oviduct, avoiding contamination of eggs intended for human consumption by Salmonella spp.
Some of the pathological changes affecting the gastrointestinal tissue include damage caused to cells producing “intestinal mucus,” which contains IgA, a type of antibody that provides local protection against bacteria. Salazar et al. (2019) evidenced that the use of β-glucans (1,3) (1,6) at a dose of 25 mg/kg for 21 consecutive days increased IgA secretion from 300 ng/mL to 2200 ng/mL. This supports the importance of reducing Salmonella spp., which causes this type of damage in the gastrointestinal tract. In this study, we performed a multiple range test and observed that the use of β-glucans reduces Salmonella spp. prevalence from 62.5% to 29.6% (P = 0.0000), consequently reducing translocation of Salmonella spp.
Science should be used as a way of improving the future of human and animal health; for this reason, we included a predicting equation (linear regression), based on which it was possible to determine the best percentage dose per ton of food for decreasing the prevalence of Salmonella spp. in the cecum of chickens (Figure 6).
As a result of the study performed, we can conclude that “the use of β-glucans (1,3) (1,6) at a dose of 25 mg/kg for 21 consecutive days reduces the population of Salmonella spp. In addition, IgA secretion increases from 300 ng/mL to 2200 ng/mL, and Salmonella spp. translocation decreases from 62.5% to 29.6% (P = 0.0000).” The predicting equation can be used as a tool.
Conclusions
Many authors have reported that SE translocation occurs from the cecum to the liver, spleen, and ovaries, resulting in severe egg yolk peritonitis, with vertical contamination of eggs intended for human consumption being the most significant consequence. The use of β-glucans (1,3) (1,6) has been proven to reduce Salmonella translocation toward these organs, especially the liver and oviduct, as it significantly increases the phagocytic activity of macrophages, consequently preventing colonization. This research shows a highly significant difference (P = 0.0000) in favor of the use of β-glucans, which decrease Salmonellas spp. colonization in the cecum by 32.93%.
It is essential to consider that the statistical model analysis presents a high variation in the decrease in bacterial colonization observed between the β-glucan-treated group (mean = 29.05 ± 9.3%; range = 19.62%–38.46%) and the control group (mean = 62.52 ± 10.21%; range = 52.5%–72.6%). This can be explained by the high heterogeneity of the selected papers. The administered dose of β-glucans (1,3) (1,6) may account for this variation. In conclusion, it can be stated that a dose of 25 mg/kg per chicken for 21 consecutive days is necessary to produce an immunomodulating effect in the bowel and a cellular immune response against Gram-negative bacteria.
Advances in bird nutrition technologies require development of new trends for imporving the efficiency of such processes. Hence, it is important to understand immunonutrition, which states that some nutrients are immunomodulators. For the two above-mentioned reasons, we aimed to build a statistical linear regression model that closely correlated with the decrease in Salmonella spp. infection with the administered dose. This equation suggests that using 1500 glucans decreases up to 18% of Salmonella spp. in the cecum of chickens.
Epilogue: Research model for Salmonella spp. translocation from the cecum to the egg intended for human consumption.
After reviewing the most important aspects of gastrointestinal immunology regarding β-glucans (1,3) (1,6) and their beneficial effects and considering the immunological changes in modern birds, further research should be performed to study the process of Salmonella spp. infection of the cecum up to systemic invasion pathogenesis in the liver and spleen of chickens, which consequently results in vertically transmission to the yolk and albumin. This type of research will help elucidate the optimal way of regulating these contamination pathways.
Image: Eggs that are possibly contaminated with bacteria.
References
Barrow, P.A. 1999. Virulence of Salmonella enterica serovar Enteritidis. In: Saeed, A.M. (ed.). Salmonella enterica serovar Enteritidis in humans and animals. Iowa State University Press, Ames, USA, p. 173-181.
Berg, R.D. 1995. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. 3:149-154.
Desmidt, M., Ducatelle, R., and Haesebrouck, F. 1997. Pathogenesis of Salmonella enteritidis phage type four after experimental infection of young chickens. Vet. Microbiol. 56:99-109.
Ducoing, A.M. 2016. Diseño estadístico de experimentos. Estadística para veterinarios y zootecnistas. Newton. pp. 207.
Farrar, A.M. 2009. The application of research synthesis methods for evaluating primary research on Salmonella in broiler chickens. M.Sc. Thesis. University of Guelph, Canada.
Gast, R.K., and Beard, C.W. 1990. Production of Salmonella enteritidis-contaminated eggs by experimentally infected hens. Avian Dis. 34:438-446.
Howard, Z.R., Moore, R.W., Zabala-Diaz, I.B., Kim, W.K., Birkhold, S.G., Byrd, J.A., Kubena, L.F., Nisbet, D.J., and Ricke, S.C. 2006. In vitro survival and growth of Salmonella typhimurium inoculated on yolk membrane after long term refrigerated storage of shell eggs. J. Con. Prot. Food Saf. 1:30-34. Humphrey, T.J. 1990. Growth of Salmonellas in intact shell eggs: influence of storage temperature. Vet. Rec. 126:292.
Immerseel, V.F. 2002. Early protection against Salmonella Infection by modification of the initial host–pathogen interactions. World Poutry Sci. J. 58:501-513.
Khan-Mohammed, Z., Adesiyun, A.A., Swanston, W.H., and Chadee, D.D. 2005. Frequency and characteristics of selected enteropathogens in fecal and rectal specimens from childhood diarrhea in Trinidad: 1998-2000. Rev. Panam. salud publ. 17:170-177.
Lowry, V.K., Farnell, M.B., Ferro, P.J., Swaggerty, C.L., Bahl, A., and Kogut, M.H. 2005. Purified beta-glucan as an abiotic feed additive up-regulates the innate immune response in immature chickens against Salmonella enterica serovar Enteritidis. Int. J. Food Microbiol. 98:309-318.
Majowicz, S.E., Musto, J., Scallan, E., Angulo, F.J., Kirk, M., O’Brien, S.J., Jones, T.F., Fazil, A., and Hoekstra, R.M. 2010. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 50:882-889.
Manners, D.J., Masson, A.J., Patterson, J.C., Björndal, H., and Lindberg, B. 1973. The structure of a β-(1→6)-d-glucan from yeast cell walls. Biochem. J. 135:31-36.
Mishu, B., Griffin, P.M., Tauxe, R.V., Cameron, D.N., Hutcheson, R.H., and Schaffner, W. 1991. Salmonella enteritidis gastroenteritis transmitted by intact chicken eggs. Ann. Intern. Med. 115:190-194.
Poppe, C. 2000. Salmonella infections in the domestic fowl. In: Wray, C. and Wray, A. Salmonella in domestic animals. CA B International, New York, US, p. 107-132.
Remus, A., Hauschild, L., Andretta, I., Kipper, M., Lehnen, C.R., and Sakomura, N.K. 2014. A meta-analysis of the feed intake and growth performance of broiler chickens challenged by bacteria. Poultry Science 93:1149-1158.
Rojas, M.W., Anaya, C., Cano, L.H., Ariztizabal, B.B., Gomez, L.M., and Lopera, H.D. 2017. Mucosa del tracto digestivo. Inmunol. Rojas, v 10:218.
Salazar, J.J. 2019. Review. Respuesta del sistema inmune de las aves a él estimulo con β-glucanos 1.3/1.6. Veterinaria y Zootecnia 13. DOI: 10.17151/vetzo.2019.13.1.6.
Salazar, J.J., Urrea, G., and Ramirez, J.D. 2019. Meta análisis: efecto immunomodulador sobre la IgA, generado por los β-glucanos 1,3/1,6 (Saccharomyces cerevisiae), en Pollos de engorde, desafiados con Salmonella entérica, Veterinaria y Zootecnia. 13. DOI: 10.17151/vetzo.2019.13.1.5.
Saleh, M.A.D., Amorim, A.B., Grecco, H.A.T., Berto, D.A., Padovani, C.R., Orsi, R.D.O., and Tse, M.L.P. 2015. Int. J. Biol. Macromol. 80:659-667.
Shao, Y., Guo, Y., and Wang, Z. 2013. Β-1,3/1,6-Glucan alleviated intestinal mucosal barrier impairment of broiler chickens challenged with Salmonella enterica serovar Typhimurium. Poult. Sci. 92:1764-1773.
Shivaprasad, H.L. 2000. Fowl typhoid and pullorun disease. Rev. Sci. Tech. OIE 19:405-424.
Telzak, E.E., Budnick, L.D., Greenberg, M.S.Z., Blum, S., Shayegani, M., Benson, C.E., and Schultz, S. 1990. A nosocomial outbreak of Salmonella enteritidis infection due to the consumption of raw eggs. N. Engl. J. Med. 323:394-397.
Timoney, J.F. 1989. Egg transmission after infection of hens with Salmonella enteritidis phage type 4. Vet. Rec. 125:600-601.
Wallis, T.S. and Galyov, E.E. 2000. Molecular basis of Salmonella-induced enteritis. MicroReview. Mol. Microbiol. 36:997-1005.
Como citar: Salazar J.J., Mejía L.M. Dynamic response of decrease in Salmonella spp. in the cecum of chickens: Impact of using mananooligosaccharides and β-glucans (1,3) (1,6). Revista Veterinaria y Zootecnia. n, v. 14, n. 1, p. 00-00, 2020. http://vetzootec.ucaldas.edu.co/index.php/component/content/article?id=289. DOI: 10.17151/vetzo.2020.14.1.8
Esta obra está bajo una Licencia de Creative Commons Reconocimiento CC BY
|