|
Development of a standardized and repeatable protocol to obtain highly concentrated platelet-rich plasma in horses
RESEARCH ARTICLE
Gabriel Cuevas-Ramos1*
1 Université de Toulouse, France; This email address is being protected from spambots. You need JavaScript enabled to view it. 2 Universitat Autònoma de Barcelona, Cerdanyola del Vallès Barcelona, Spain; This email address is being protected from spambots. You need JavaScript enabled to view it. and This email address is being protected from spambots. You need JavaScript enabled to view it. 3 Fidia, Madrid, Spain; This email address is being protected from spambots. You need JavaScript enabled to view it. * Correspondence: This email address is being protected from spambots. You need JavaScript enabled to view it., Gabriel Cuevas Ramos. Current address: Large Animal Teaching Hospital, University of Copenhagen, Agrovej 8, 2630 Taastrup, Denmark. Phone: 00 45 51808598
Received: 17 december 2018 and Approved: 30 may 2019, Updated: 17 june 2019
DOI: 10.17151/vetzo.2019.13.2.5
SUMMARY. Introduction: Platelet-rich plasma (PRP) is an autologous blood-derived product with several therapeutic applications. Platelet growth factors have been shown to stimulate healing and reduce pain. Its use in the equine industry, particularly for orthopedic problems such as osteoarthritis or tendonitis has been increasing. However, PRP lacks reproducibility and, sometimes, quality since not only individual (breed, age, gender) and environmental (time of the day, hydration status) factors, but also laboratory methods can affect its final quality. Aims: we aimed to develop and normalize a protocol for PRP preparation, which will be repeatable for equine use, through a sterile disposable kit with a standard centrifuge, which minimizes laboratory variants. Methods and results: The final protocol resulting in a PRP with very high platelet concentration (6.4 ± 1.3 fold increase) and low white blood cell counts (3.7 ± 1.8 x106/mL) was stablished. The usual fold increase reported is 2 to 3. The final PRP had concentrations of platelet-derived growth factor with an average of 37ng/ml. Conclusion: The protocol is highly repeatable and simple to perform and the kit is suitable to use under field conditions because it does not permit contact between the sample and ambient air along the process.
Keywords: platelet-rich plasma; horses; new method
Desarrollo de un protocolo estandarizado y repetible para obtener plasma altamente concentrado rico en plaquetas en caballos
RESÚMEN. Introducción: el plasma rico en plaquetas (PRP) es un producto autólogo derivado de la sangre con varias aplicaciones terapéuticas. Se ha demostrado que los factores de crecimiento plaquetario estimulan la curación y reducen el dolor. Su uso en la industria equina, particularmente para problemas ortopédicos como la osteoartritis o la tendinitis ha ido en aumento. Sin embargo, el PRP carece de reproducibilidad y a veces, de calidad, ya que no solo los factores individuales (raza, edad, sexo) y ambientales (hora del día, estado de hidratación), sino también los métodos de laboratorio pueden afectar su calidad final. Objetivos: nuestro objetivo fue desarrollar y normalizar un protocolo para la preparación de PRP, que será repetible para uso equino, a través de un kit desechable estéril con una centrífuga estándar, que minimiza las variantes de laboratorio. Métodos y resultados: se estableció el protocolo final que resultó en un PRP con una concentración de plaquetas muy alta (6,4±1,3 veces mayor) y recuentos bajos de glóbulos blancos (3,7±1,8 x106/ml). El PRP final tenía concentraciones de factor de crecimiento derivado de plaquetas con un promedio de 37 ng/ml. Conclusión: El protocolo es altamente repetible y simple de realizar, y el kit es adecuado para usar en condiciones de campo porque no permite el contacto entre la muestra y el aire ambiente a lo largo del proceso.
Palabras clave: plasma rico en plaquetas; caballos, nuevo método
Introduction
Platelet-rich plasma (PRP) is a biological product first introduced in regenerative medicine around thirty years ago; its earliest documented uses are treatment of cardiac disease, dental damage, and maxillofacial surgery [1,2]. Its use in the equine industry, particularly for orthopedic problems such as osteoarthritis or tendonitis treatment, has been increasing [3,4]. PRP is an autologous blood-derived product created by obtaining a small amount of blood and concentrating platelets generally through centrifugation (one or two steps). The usual fold increase reported in the literature is two to three times more than the normal levels on circulating blood [5].
Platelets degranulate after exposure to collagen or when activated with calcium or thrombin. Alpha granules then release more than 300 different molecules [6,7] including platelet-derived growth factors (PDGF), transforming growth factors β1 (TGFβ1), insulin-like growth factors (IGF), platelet factor 4 (PF-4), fibroblast growth factor 2 (FGF-2), and vascular endothelial growth factor (VEGF). These factors are known to potentially accelerate healing and promote cartilage repair [8–10], inhibit chondrocyte apoptosis, stimulate bone and vessel remodeling and collagen synthesis, modulate inflammation [7], and stimulate excretion of anti-inflammatory cytokines such as interleukin 4 (IL-4) and the receptor antagonist of IL-1(IL-1ra). The degranulation process is quick with around 70% of the growth factors released within 10 min [11,12], but sustained growth factor release has been observed for up to 8 days in vitro [1].
For various reasons, PRP can lack reproducibility and quality. In horses, individual factors (breed, age, gender) [13] and environmental factors (time of the day, hydration status) [14] have been demonstrated to play a role in final PRP composition. Additionally, laboratory preparation methods can also affect the final quality of PRP [15]. Not only the type of centrifuge could be a cause, but also the material used and the environment where PRP is prepared; therefore, extreme care should be taken to avoid contamination, particularly if the product is injected, as it is often the case in equine orthopedic applications [3]. To better control laboratory factors, the use of a laminar flow chamber or at least clean ambient air conditions, and trained sanitary personnel to prepare PRP have been suggested [15]. Hence, it can be sometimes difficult to control individual and/or environmental factors that could affect the final product components, but using standardized equipment and protocols could help produce a PRP with more constant characteristics and quality.
The objective of this work was to develop, optimize and standardize a protocol that would be repeatable, for equine use, in terms of centrifuge speed, time, and volume for PRP preparation, using a commercial closed disposable kit.
Materials and Methods
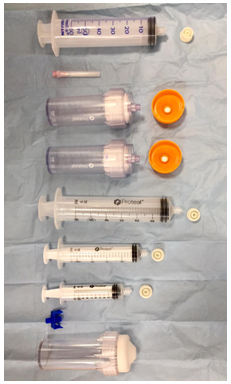
Fidia® provided the commercial kit. It is based on a closed system, which means that while processing the blood, the sample does not have any contact with ambient air. The kit is presented fully sterile and contains all the necessary components (Figure 1) to allow sample processing for obtaining PRP using a double spin method. The centrifuge used for the study was a Duo-Grafter II, also provided by Fidia®. Firstly, five healthy research horses were used for the optimization process; 52 ml of blood with sodium citrate 3.8 % as anticoagulant, were collected two times from each horse, using sterile asepsis and gloves, and then transferred to a 50ml processing tube included in the kit. Blood samples were taken in the morning, at the same clock time (10:00 a.m.). After the first centrifugation, the whole plasmatic fraction (PRP-1) was recovered using one of the syringes provided in the kit. The tubes in the kit allow aspirating the sample without the need of actually opening the tube (Figure 1). PRP-1 was then transferred to a second 50ml tube for the second spin step. After the second centrifugation, the top two thirds of the plasma were considered the platelet-poor plasma (PPP-2) fraction [16]. The remaining lower third of the plasma was considered the final platelet-rich plasma (PRP-2); it was aspirated from the tube with a lower-lock syringe, maintaining the close system characteristics of the kit, and set on a final volume of 6ml. For the first centrifugation step, five different speeds were evaluated: 1100, 1300, 1400, 1500 and 1700 revolutions per minute (rpm), and four conditions were evaluated for the second spinning (1500, 1700, 2000, 2300 rpm), all spins were set at 8 minutes. Platelets, red blood cells, and white blood cells (WBC) counts were done for each centrifugation step using a cell counter (BioQControl, Fidia®). 1ml sample of each centrifugation step, PPP and PRP, was immediately frozen at -20°C for further growth factor analysis. Platelet increase folds were calculated as the ratio obtained between the platelet concentration on the PRP1 or PRP2, and the initial values on circulating blood. Optimal centrifugation conditions were established when the sample (PRP-1 or PRP-2) reached the maximum possible platelets counts and the lowest possible WBC. Secondly, a validation of the process was done through the repeatability examination of the method, using five more horses, repeating all the above mentioned steps, with a fixed first spin speed at 1100rpm for 8 minutes, and a second centrifugation speed fixed at 2100rpm for 8 minutes. Quantification of PDGF subunit A concentration was measured in plasma and PRP-2 using an equine specific sandwich ELISA technique (MyBiosource MBS040792). The day of the analysis, the samples were defrosted, thawed at room temperature, and centrifuged. The supernatant was used for growth factor analysis. Statistical analysis was done using the Wilcoxon test for non-parametric data, and the Mann-Whitney U test for the parametric data, using the GraphPad Prism version 7 software.
Results
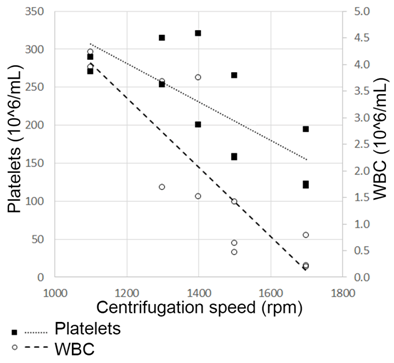
The increase on the centrifugation speed from 1100 up to 1700 rpm showed a different kinetic pattern between WBC and platelets. Higher counts of WBC and platelets were obtained at lower rpm. Based on this pattern, a commitment value of 1550rpm was reached to select a PRP-1 with a leucocyte concentration of less than 1.5 x 106/ml and a platelets concentration of more than 200x106/ml (Figure 2). The platelet concentration fold factor correlated negatively to the rpm (p=0.1557; CI 95% -0.0054 to -0.0007), meaning that a too low speed showed up to give high concentrations of platelets and of WBC. On the contrary, too high spin speeds caused platelet aggregates even though WBC counts were very low. A point in which a good platelet count was obtained with a low WBC count was then chosen.
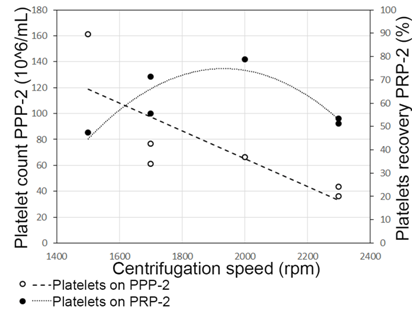
Following the same approach, the second centrifugation parameters were set at 2100 rpm. The PPP fraction had a very low platelet concentration (<60x106/ml), which corresponded to around 30% of total platelets counted on the second spin. PRP-2 was obtained on a final 6ml volume, with platelet, WBC and red blood cells average concentrations of 756±143 x106/mL; 3.7 ± 1.8 x106/mL; 0.01±0.01 x109/ml, respectively. Using these settings, PRP-2 contained in average 70% of total platelets counted (Figure 3). The mean fold platelet concentration increase obtained was 6.4±1.3. Growth factor analysis resulted in PRP-2 having a PDGF mean concentration of 37±3 ng/ml, which represented a significant difference in comparison with PDGF levels on plasma (12ng/mL) (p = 0.03).
Figure 1. Sterile kit components. The kit provided by Fidia® contains two Push-Out® 50ml tubes with orange caps. These tubes allow injecting, with a needle, the sample inside the tube and bypassing the push-out system needed for extraction. After centrifugation, PRP-1 was recovered with a 50ml syringe by placing it over the threaded lock on top of the Push-Out tube, and simply aspirating the sample. PRP-1 was then transferred, by injection, to the second Push-out orange-cap tube. PPP-2 was recovered by aspiration with the 10ml syringe, and the PRP-2 was recovered with the 5ml syringe. Therefore, the sample never has contact with ambient air. The whitecap tube is provided for tare the Duo-Grafter II centrifuge.
Figure 2. Determination of first spin centrifugation speed. Platelet and white blood cell counts were done at five different spin speeds. Cell counts are expressed on 106/ml. 1550rpm was stablished as an adequate point to collect PRP-1, with a WBC concentration of less than 1.5x106/ml and a platelets concentration of more than 200x106/ml. Higher spin speeds caused platelet aggregates even though WBC counts were lower. In contrast, slower centrifugation speeds lead to higher platelets counts, but also to higher WBC concentrations.
Figure 3. Total platelet recovery on PRP-2 versus PPP-2. By setting 2100rpm as the second centrifugation speed, PPP-2 contained less than 60x106/mL of platelets, which represented <30% of the total platelet count (PPP-2+PRP-2). Hence, these settings allow us to recover an average of 70% of total platelets (756±143 x106/ml) in PRP-2.
Discussion
In humans, the positive clinical effects of PRP have been studied and validated in different conditions and procedures, such as knee osteoarthritis, total knee arthroplasty, meniscal repair, and collateral ligament reconstruction [17]. In horses, previous studies have demonstrated beneficial therapeutic effects of PRP for treatment of refractory fetlock osteoarthritis [3] or musculoskeletal trauma [4]. PDGFs are thought to be in part responsible for the healing effects of PRP [18,19]. For instance, PDGF will promote cell proliferation and migration in specific tissues depending on the isoform (A or B) and receptor expressed on the target tissue; PDGF-AA and PDGF-BB will target mesenchymal stem cells to promote proliferation and angiogenesis, and PDGF-AA can enhance osteogenesis and bone regeneration [20]. The final product (PRP-2) obtained in this study contained significantly higher levels of this growth factor when compared with PRP-1.
In humans, the currently accepted platelet concentration of PRP is 4-5 fold compared to the basal blood sample [17,21]. Acceptable platelet counts in equine PRP has been suggested to be 250-500x106/ml, which represents a fold increase of around two to three times [5], considering an average platelet count on blood of 100-350x106/ml [22]. Clinical studies are needed to determine if the very high platelet concentrations (756 ± 143 x106/ml) and the low WBC count (3.7 ± 1.8 x106/mL) presented here, make the product more efficacious. Benefits of high platelet concentrations in PRP are currently not entirely clear [23], although highly concentrated PRP has been associated with stimulation of bone regeneration [24]. Nevertheless, in equine orthopedics, the importance of fold increase and/or the absolute number of platelets has not been fully explored, as well as the optimal concentration of WBC, which may also differ according to the clinical application itself [14,25]. The PRP obtained through the proposed methodology falls within the classification of a PRP leuko-reduced [16,26]. An elevated number of WBC could increase pro-inflammatory cytokines concentration and release of enzymes, such as matrix metalloproteinases, which might have antagonistic effects [27]. Superior anabolic and anti-inflammatory effects of leuko-reduced PRP in treatment of osteoarthritis [3,7] and tendonitis [28] have been observed. In humans, highly concentrated PRP has been linked with positive stimulation on bone regeneration [24,29]. Further research is needed to fully understand in which equine clinical applications we need higher or lower platelets concentrations, and/or leuko-reduced or leuko-rich PRPs.
Another advantage of the method described here is the closed system characteristics of the kit. Even if it has been reported that it is not mandatory to produce PRP under a laminar flow cabinet or a Bunsen burner, the need of a laboratory environment have been recommended [30]. The kit presented here is suitable to use in fieldwork because the sample has no contact with ambient air until the final product is obtained. This is important as many equine practitioners work on ambulatory practices and they cannot easily get access to a room with clean laboratory conditions.
Conclusions
This study shows the establishment of a standardized method to obtain highly concentrated (6.4 ± 1.3 increase fold), leuko-reduced PRP, using a simple closed kit. Platelets counts were repeatable in the examined equine samples. The kit does not permit contact between the sample and the ambient air, therefore, together with the small size centrifuge, it is easy to use not only in hospitals but also for ambulatory practices.
Author Contributions: GCR, LV and MP developed the study. GCR, DA and MP wrote and corrected the article. LV provided the commercial kit and centrifuge. Funding: This research received no external funding. Conflicts of Interest: The authors declare no conflict of interest.
References
Alvarez, M. E., Giraldo, C. E., & Carmona, J. U. (2010). Monitoring bacterial contamination in equine platelet concentrates obtained by the tube method in a clean laboratory environment under three different technical conditions. Equine Veterinary Journal, 42(1), 63–67. https://doi.org/10.2746/042516409X455221 PMID:20121916
Andia, I., and Abate, M. (2014). Knee osteoarthritis: Hyaluronic acid, platelet-rich plasma or both in association? Expert Opinion on Biological Therapy, 14(5), 635–649. https://doi.org/10.1517/14712598.2014.889677 PMID:24533435
Andia, I., and Maffulli, N. (2013). Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nature Reviews. Rheumatology, 9(12), 721–730. https://doi.org/10.1038/nrrheum.2013.141 PMID:24080861
Anitua, E., Zalduendo, M. M., Alkhraisat, M. H. and Orive, G. (2013). Release kinetics of platelet-derived and plasma-derived growth factors from autologous plasma rich in growth factors. Annals of Anatomy, 195(5), 461–466. https://doi.org/10.1016/j.aanat.2013.04.004 PMID:23722041
Argüelles, D., Carmona, J. U., Pastor, J., Iborra, A., Viñals, L., Martínez, P., Prades, M. (2006). Evaluation of single and double centrifugation tube methods for concentrating equine platelets. Research in Veterinary Science, 81(2), 237–245. https://doi.org/10.1016/j.rvsc.2005.12.008
Carmona, J. U., and López, C. (2011). Autologous Platelet Concentrates as a Treatment for Shoulder Injury in a Horse. Journal of Equine Veterinary Science, 31(9), 506–510. https://doi.org/10.1016/j.jevs.2011.03.008
Cerciello, S., Beitzel, K., Howlett, N., Russell, R. P., Apostolakos, J., McCarthy, M. B., Mazzocca, A. D. (2013). The Use of Platelet-Rich Plasma Preparations in the Treatment of Musculoskeletal Injuries in Orthopaedic Sports Medicine. Operative Techniques in Orthopaedics, 23(2), 69–74. https://doi.org/10.1053/j.oto.2013.07.001
Coppinger, J. A., Cagney, G., Toomey, S., Kislinger, T., Belton, O., McRedmond, J. P., Maguire, P. B. (2004). Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood,103(6), 2096–2104. https://doi.org/10.1182/blood-2003-08-2804 PMID:14630798
Dohan Ehrenfest, D. M., Bielecki, T., Mishra, A., Borzini, P., Inchingolo, F., Sammartino, G., Everts, P. A. (2012). In search of a consensus terminology in the field of platelet concentrates for surgical use: Platelet-rich plasma (PRP), platelet-rich fibrin (PRF), fibrin gel polymerization and leukocytes. Current Pharmaceutical Biotechnology, 13(7), 1131–1137. https://doi.org/10.2174/138920112800624328 PMID:21740379
Dohan Ehrenfest, D. M., Rasmusson, L., and Albrektsson, T. (2009). Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends in Biotechnology, 27(3), 158–167. https://doi.org/10.1016/j.tibtech.2008.11.009
Fontenot RL, Sink CA, Werre SR, Weinstein NM, Dahlgren LA. Simple tube centrifugation for processing platelet-rich plasma in the horse. Can Vet J = La Rev Vet Can 2012;53:1266–72.
Fortier, L. A., Barker, J. U., Strauss, E. J., McCarrel, T. M., and Cole, B. J. (2011). The Role of Growth Factors in Cartilage Repair. Clinical Orthopaedics and Related Research, 469(10), 2706–2715. https://doi.org/10.1007/s11999-011-1857-3
Giraldo, C. E., López, C., Álvarez, M. E., Samudio, I. J., Prades, M., and Carmona, J. U. (2013). Effects of the breed, sex and age on cellular content and growth factor release from equine pure-platelet rich plasma and pure-platelet rich gel. BMC Veterinary Research, 9(1), 29. https://doi.org/10.1186/1746-6148-9-29
Graziani, F., Ivanovski, S., Cei, S., Ducci, F., Tonetti, M., and Gabriele, M. (2006). The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clinical Oral Implants Research, 17(2), 212–219. https://doi.org/10.1111/j.1600-0501.2005.01203.x PMID:16584418
Holmes, H. L., Wilson, B., Goerger, J. P., Silverberg, J. L., Cohen, I., Zipfel, W. R., and Fortier, L. A. (2018). Facilitated recruitment of mesenchymal stromal cells by bone marrow concentrate and platelet rich plasma. PLoS One, 13(3), e0194567. https://doi.org/10.1371/journal.pone.0194567
Kawasumi, M., Kitoh, H., Siwicka, K. A., and Ishiguro, N. (2008). The effect of the platelet concentration in platelet-rich plasma gel on the regeneration of bone. The Journal of Bone and Joint Surgery. British Volume, 90(7), 966–972. https://doi.org/10.1302/0301-620X.90B7.20235 PMID:18591611
Li, A., Xia, X., Yeh, J., Kua, H., Liu, H., Mishina, Y., Li, B. (2014). PDGF-AA promotes osteogenic differentiation and migration of mesenchymal stem cell by down-regulating PDGFRα and derepressing BMP-Smad1/5/8 signaling. PLoS One, 9(12), e113785. https://doi.org/10.1371/journal.pone.0113785
Magalon, J., Bausset, O., Serratrice, N., Giraudo, L., Aboudou, H., Veran, J., Sabatier, F. (2014). Characterization and Comparison of 5 Platelet-Rich Plasma Preparations in a Single-Donor Model. Arthrosc J Arthrosc Relat Surg, 30(5), 629–638. https://doi.org/10.1016/j.arthro.2014.02.020
Marx, R. E. (2004). Platelet-rich plasma: Evidence to support its use. Journal of Oral and Maxillofacial Surgery, 62(4), 489–496. https://doi.org/10.1016/j.joms.2003.12.003 PMID:15085519
Marx, R. E., Carlson, E. R., Eichstaedt, R. M., Schimmele, S. R., Strauss, J. E., and Georgeff, K. R. (1998). Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics, 85(6), 638–646. https://doi.org/10.1016/S1079-2104(98)90029-4 PMID:9638695
McCarrel TM., Minas T., Fortier, LA. (2012). Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Joint Surg Am. 94:e143(1-8). doi:10.2106/JBJS.L.00019.
O’Shea, C. M., Werre, S. R., and Dahlgren, L. A. (2015). Comparison of platelet counting technologies in equine platelet concentrates. Veterinary Surgery, 44(3), 304–313. https://doi.org/10.1111/j.1532-950X.2014.12290.x PMID:25307726
Pichereau, F., Décory, M., and Cuevas Ramos, G. (2014). Autologous platelet concentrate as a treatment for horses with refractory fetlock osteoarthritis. Journal of Equine Veterinary Science, 34(4), 489–493. https://doi.org/10.1016/j.jevs.2013.10.004
Rinnovati, R., Romagnoli, N., Gentilini, F., Lambertini, C., and Spadari, A. (2015). The influence of environmental variables on platelet concentration in horse platelet-rich plasma. Acta Veterinaria Scandinavica, 58(1), 45. https://doi.org/10.1186/s13028-016-0226-3
Satué, K., Gardón, J., and Muñoz, A. (2017). Interpretation of Platelets in The Horse. Journal of Hematology Research, 4, 19–25. https://doi.org/10.12974/2312-5411.2017.04.3
Spaková, T., Rosocha, J., Lacko, M., Harvanová, D., and Gharaibeh, A. (2012). Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. American Journal of Physical Medicine & Rehabilitation, 91(5), 411–417. https://doi.org/10.1097/PHM.0b013e3182aab72
Sundman, E. A., Cole, B. J., and Fortier, L. A. (2011). Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. The American Journal of Sports Medicine, 39(10), 2135–2140. https://doi.org/10.1177/0363546511417792 PMID:21846925
Sundman, E. A., Cole, B. J., Karas, V., Della Valle, C., Tetreault, M. W., Mohammed, H. O., and Fortier, L. A. (2014). The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. The American Journal of Sports Medicine, 42(1), 35–41. https://doi.org/10.1177/0363546513507766 PMID:24192391
Wasserman, A., Matthewson, G., and MacDonald, P. (2018). Platelet-Rich Plasma and the Knee-Applications in Orthopedic Surgery. Current Reviews in Musculoskeletal Medicine, 11(4), 607–615. https://doi.org/10.1007/s12178-018-9521-0
Zhu, Y., Yuan, M., Meng, H. Y., Wang, A. Y., Guo, Q. Y., Wang, Y., and Peng, J. (2013). Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: A review. Osteoarthritis and Cartilage, 21(11), 1627–1637. https://doi.org/10.1016/j.joca.2013.07.017 PMID:23933379
Como citar: Cuevas-Ramos, G.; Arguelles, D.; Vidal, L.; Prades, M. Development of a standardized and repeatable protocol to obtain highly concentrated platelet-rich plasma in horses. Revista Veterinaria y Zootecnia, v. 13, n. 2, p. 52-62, 2019. http:// vetzootec.ucaldas.edu.co/index.php/component/content/article?id=276. DOI: 10.17151/vetzo.2019.13.2.5
Esta obra está bajo una Licencia de Creative Commons Reconocimiento CC BY
|